image text translation
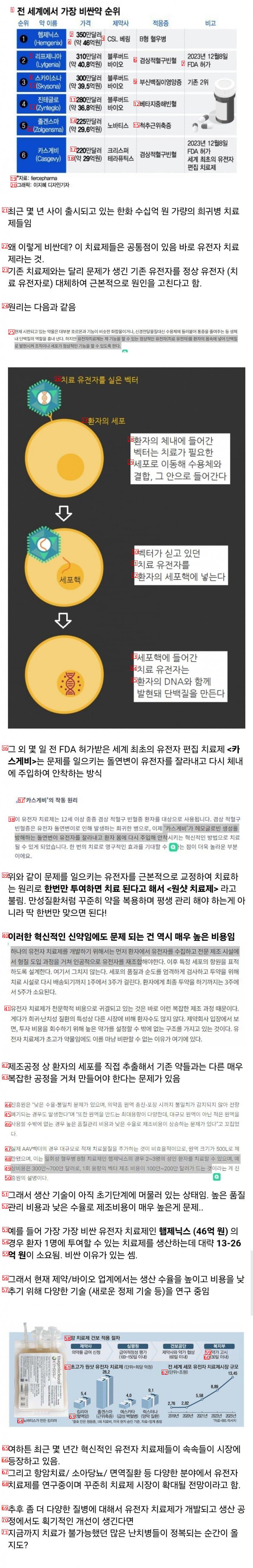
(1)World’s Most Expensive Drug Ranked Drug Name Price
(2)$3.5 million
(3)CSL Bering Type B hemophilia
(4)about 4.6 billion won
(5)Leaf Genia $3.1 million Bluebird December 8, 2023
(6)sickle cell anemia
(7)FDA Approved Lyfgenia Approximately KRW 40.8 Billion Bio
(8)Skysona, $3 million
(9)Adrenal Protein Dystrophy Ranked 2nd
(10)Skysona about 39.5 billion won
(11)$2.8 million
(12)Beta Mediterranean anemia
(13)Zynteglo about 36.8 billion won
(14)$2.25 million
(15)spinal muscular dystrophy
(16)Zolgensma about 29.6 billion won
(17)$2.2M CRISPR FDA Approved World’s First Gene Editing Treatment
(18)About 2.9 billion won Therapeutics
(19)자료 fiercepharma
(20)Graphic Lee Jihye Design Reporter
(21)These are rare disease treatments worth billions of won that have been released in recent years
(22)Why is it so expensive? What these treatments have in common is that they are gene therapy
(23)Unlike conventional treatments, it is said that the cause is fundamentally corrected by replacing the existing gene in question with a normal gene therapy gene
(24)The principle is as follows
(25)Most drugs currently on the market mimic the role of proteins in the body, such as compounds that have similar functions to hormones or attach to receptors instead of neurotransmitters to reduce pain. However, gene therapy drugs put normal gene therapy genes that can function properly into the patient’s body and express them as proteins, allowing tissues or cells to function normally
(26)a vector carrying the therapeutic gene
(27)a patient’s cell
(28)The vectors that enter the patient’s body need to be treated
(29)It moves into the cell, binds to the receptor, and enters it
(30)Vector was carrying
(31)The treatment gene
(32)Put it in the patient’s cell nucleus
(33)in the nucleus of a cell
(34)The treatment gene is
(35)It is expressed with the patient’s DNA to make protein
(36)The world’s first gene-editing drug, Kasgevi, was approved by the FDA a few days ago to cut off the mutated gene that causes the problem and inject it back into the body to settle
(37)How Kasgevi Works
(38)This gene therapy is used for patients with severe sickle cell anemia over the age of 12 Sickle erythrocytic anemia is a rare disease caused by genetic mutations and can now be treated in an innovative way in which Kasgevi cuts down mutated genes that interfere with hemoglobin production and injects them back into the patient’s body to settle What’s even more surprising is that one treatment can expect a permanent effect
(39)With the principle of fundamentally correcting and treating genes that cause problems as above, just because it is treated once, it does not mean that you have to take the drug steadily and manage it for life like a chronic disease called a one-shot treatment, but only once!
(40)Despite being such an innovative new drug, it is also very expensive, but in order to develop a gene therapy, it is necessary to first collect genes from patients and artificially recombine genes through transgenic introduction at specialized manufacturing facilities After that, it is designed to target the antigen of a specific cell. It does not stop there. It takes one to three weeks for the quality and purity of the cell to be rigorously tested and shipped back to the treatment facility for medication It takes three to five weeks for the final dose to be given to the patient
(41)It is because of this complicated manufacturing process that gene therapy is resulting in astronomical costsMoreover, due to the nature of rare and intractable diseases, the number of patients is not as high as in other markets From the pharmaceutical company’s point of view, it has a structure in which it has no choice but to set high drug prices to recover investment costs This is why we can’t criticize the gene therapy drug even though it’s an expensive drug
(42)It extracts the patient’s cells directly from the manufacturing process, and it’s very different from the existing drugs
(43)There is a problem that it has to be made through a complex process
(44)The agency has a low yield and inconsistency problem, and no discrepancy is detected until drug stock is filled and packaged
(45)There are cases where it is discarded, and the maximum capacity of making undiluted solution varies, but a small amount of undiluted solution is not a large-scale undiluted solution
(46)It was pointed out that there is a problem of increasing manufacturing costs due to high quality management costs and low yield when it has no choice but to use it
(47)For the actual AAV vector, it is inefficient to add a large-scale load treatment substance, so the stock solution size is 500L
(48)It’s a one-time hemophilia type B treatment, Hemgenix, can treat two to three adult patients. Yes
(49)The truth is that 300 for standing costs between $10,000 and $7 million, which costs between $1 million and $2 million to manufacture vectors for a single capacity
(50)It’s an explanation of the excitement
(51)So the production technology is still in its infancy. High quality
(52)The problem is that manufacturing costs are very high due to management costs and low yield
(53)For example, the most expensive gene therapy drug, Hamgenix, is 4.6 billion won
(54)In this case, it produces a treatment that can be administered to one patient, approximately 13-26 characters
(55)There’s a reason why it costs hundreds of millions of won
(56)In the current pharmaceutical bio industry, production yields are raised and costs are lowered
(57)I’m studying various technologies, new refining technologies, and so on
(58)Procedures for applying cancer treatment health insurance
(59)Pharmaceutical company and drug price negotiation drug benefit application for drug price evaluation suitability evaluation
(60)Within 30 days, 100 to 150 days, 60 days
(61)Global cell gene therapy market size of KRW 100 million per unit of ultra-high-priced one-shot gene therapy
(62)Unit = trillion won
(63)Blood cancer muscular dystrophy Acute Leukemia Retinal Diseases 2019 2021 2023 2025
(64)Kimria Data made by Novartis = The BBS Research Pal proposal is a comprehensive data industry based on the U.S. local approval for one treatment for Jeongyong’s disease
(65)In any case, over the last few years, innovative myogenetic treatments and drugs have come into the market
(66)It’s here
(67)And genes in various fields such as chemotherapy, childhood diabetes, and immune diseases
(68)The therapeutic market is expected to expand as treatment research is conducted steadily
(69)In the future, gene therapy drugs will be developed and produced for more diverse diseases
(70)If a groundbreaking dog gets a bull’s eye in Jeong
(71)So far, the moment when many incurable diseases were conquered came
!