I was so excited and excited since yesterday
There are overwhelming opinions that I don’t understand
That’s why you can’t talk about this on the table
Feel it and start writing information with the lowest level of difficulty
Especially when I talked about CDW, I saw that the reactions were getting intense
Properties vary depending on the atomic arrangement structure even if it consists of components such as pre-summary
1 Decision Crystal
Atoms come together to form matter, usually a crystal is made
Considering that the atomic arrangement structure is made into a periodic repetition
To make it easier for the general public to understand this
The atoms are arranged like tiles on the floor of the building
Think about it

The bathroom tile square shape is repeated
But even if tiles are laid, there is not only one shape of tiles that can be laid repeatedly

Like this
The area of this is crystalline structure, and not only physics, but also chemistry, chemical engineering, and new materials
The repeating unit that anyone learns of this is called the lattice lattice itself, and the arrangement of atoms that make up a lattice is called the base base
to put it simply
The repeated tile itself is a grid and the shape of the tile can be considered as the base
Tile from a two-dimensional perspective and brick from a three-dimensional perspective
The reason for studying the crystal structure is that it is the basis for understanding the properties of matter
The easiest example is a material that is composed of elements like carbon allotropes but has different structures
The famous allotropes of carbon are fullerene C60 carbon nanotubes CNT graphene graphene graphite diamond diamond diamond jewelry rings
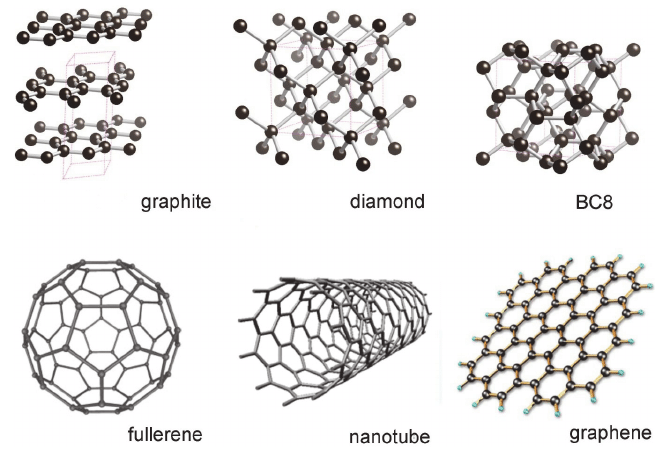
All of the atomic arrangements of carbon isotopes are composed of only carbon atoms, and surprisingly, even these are only a few
Image Source
httpstestbookcomquestion-answerwhich-of-the-following-is-an-allotropic-form-of-ca–614d7651abb6486c95796411
The interesting fact is that the properties of these carbon isotopes are different properties
First, carbon nanotube graphene graphite has the properties of metal in itself

The pencil lead is an electric conductor because it is made of graphite
But the jewelry diamonds sold at DeViers are non-conductors

Diamond, a material that has been proven to consist only of carbon but is not conductive
You’ll feel it when you see it They’re made up of only carbon, but they have different properties
That’s right.
As a result, the tile brick method in which carbon atoms are arranged is different
Modern solid-state physics begins with understanding these atomic arrangement structures